Sodium chloride lead II nitrate lead II chloride sodium nitrate. Aqueous sodium chloride reacts with aqueous lead II nitrate to yield a lead II chloride precipitate and aqueous sodium nitrate.

How To Write The Net Ionic Equation For Pb No3 2 Nacl Youtube
PH of NaCl and AgNO 3 reaction Both NaCl and AgNO 3 are neutral solutions.

. CPb2 aq2Cl- aqPbCl s. Click in the answer box to open the symbol palette. Aqueous sodium chloride reacts with aqueous lead II nitrate to yield a lead II chloride precipitate and aqueous sodium nitrate sodium chloride lead II nitrate lead II chloride sodium nitrate NaCl PbNO 3 2 PbCl 2 NaNO 3 2 NaCl aq PbNO 3 2 aq PbCl 2 ppt 2 NaNO 3 aq 2.
C State the physical conditions of reactants in which the reaction will not take place. Click to see full answer. Therefore you can see AgCl is precipitated in the solution.
The chemical equation for this reaction is. What is the net ionic equation for this reaction. 2 SO 4 aq to yield a.
Ba NO32 aq H2SO4 - BaSO4 downward arrow 2HNO3 aq Hope this help. When sodium chloride solution is added to lead nitrate solution then it results in the formation of a precipitate of lead chloride and sodium nitrate. Once you know how many of each type of atom you can only change the coefficients the numbers in front of atoms or compounds to balance the equation for Lead II nitrate Sodium chloride.
See the answer Select the precipitate that forms when aqueous lead II nitrate reacts with aqueous sodium chloride. 2 NaCl PbNO32 PbCl2 2 NaNO3 Type of Reaction. And all halides are soluble EXCEPT for AgX P bX2 and H g2X2.
LeadII nitrate reacts with sodium chloride in aqueous solution to form a precipitate. The products of the reaction are AgCl and NaNO 3. All nitrates are soluble hence silver nitrate is soluble.
The precipitate lead chloride is insoluble in cold water but it is soluble in hot water. D Name the type of chemical reaction which occurs. Aqueous barium nitrate reacts with sulfuric acid H 2 SO 4.
The reaction equation for this chemical reaction is as follows. The chemical equations for. 3 2 NaCl aq PbNO.
CoI2 aq Pb NO32 aq PbI2 s Co NO32 aq Give the balanced ionic equation for the reaction. 2NaClaq ZnOH2s Refer to the related link below. Balance the equation and classify it.
Aqueous ammonium chloride and aqueous leadII nitrate reacts to form solid leadII chloride and aqueous ammonium nitrate Write the molecular equation the complete ionic equation and the net ionic equation for this reaction_ CS Courtney S. B Write the balanced chemical equation for the reaction which takes place. What is the balanced equation for aqueous ammonium chloride and aqueous lead II nitrate.
3 2 PbCl 2 NaNO. Click to see full answer. ZnCl2aq 2NaOHaq --.
BaNO32 aq H2SO4 - BaSO4 downward arrow 2HNO3 aq Hope this help. Aqueous barium nitrate reacts with sulfuric acid H. The reaction of aqueous cobalt II iodide and aqueous lead II nitrate is represented by the balanced formula equation.
The example shown in the figure is not the same as described in the question. 1 Answer anor277 Nov 8 2016 AgN O3aq N aClaq N aN O3aq AgCls Explanation. - Answers 2NH4Cl aq PbNO32 aq ---- 2NH4NO3 aq PbCl2 s Home Subjects Math Science History.
Chemistry Enter the net ionic equation for the reaction of aqueous sodium chloride with aqueous silver nitrate ℹ ej Oct 11 2010 AgNO3 aq NaCl aq AgCl s NaNO3 aq is the molecular equation. PbNa 2 If the molality of a solution of NaCl aq is 170 m what is the percent by mass of NaCl in the solution. Ag aq Cl -aq 25 10 -3 mol 2 dm -6 Ksp AgCl 17 10 -10 mol 2 dm -6 Because calculated value for Ksp expression is greater than Ksp value of AgCl.
Na 2 NO 3 c. Write a balanced equation to describe this reaction. Aqueous sodium chloride reacts with aqueous lead nitrate to yield a lead chloride precipitate and aqueous sodium nitrate are.
Aqueous zinc chloride plus aqueous sodium hydroxide produce aqueous sodium chloride and solid zinc hydroxide. LeadII nitrate PbNO₃₂ aq Sodium chloride NaCl aq The products are. Aqueous ammonium chromate reacts with aqueous leadII nitrate in a double-displacement reaction.
A What happens when an aqueous solution of sodium sulphate reacts with an aqueous solution of barium chloride. Aqueous sodium chloride reacts with aqueous lead nitrate to yield a lead chloride precipitate and aqueous sodium nitrate are. Include states of matter in your answer.
The chloride makes a precipitate with the Pb². LeadII chloride PbCl₂ s Sodium nitrate NaNO₃ aq Salts form nitrate are soluble. To balance PbNO32 NaCl PbCl2 NaNO3 youll need to be sure to count all of atoms on each side of the chemical equation.
3 2 aq PbCl 2 ppt 2 NaNO 3 aq2. Net ionic is Ag aq Cl- aq AgCl s ℹ DrBob222 Oct 11 2010 iight thanx ℹ ej Oct 11 2010. Cl 2 NO 3 d.
This reaction is commonly used to illustrate basic solubility rules and solubility equilibria. Aqueous sodium chloride reacts with aquaeous leadII nitrate to yield a leadII chloride precipitate and aqueous sodium nitrate. PbNO₃₂ aq 2NaCl aq PbCl₂ s 2NaNO₃ aq.
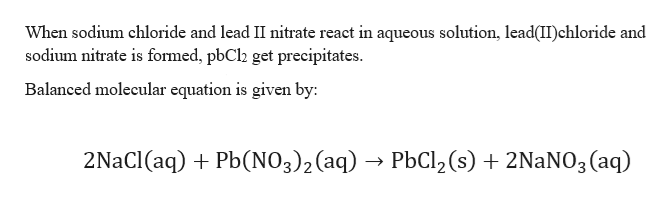
Answered When Sodium Chloride And Lead Ii Bartleby

0 Comments